Artificial Photosynthesis
By Chris Goncher
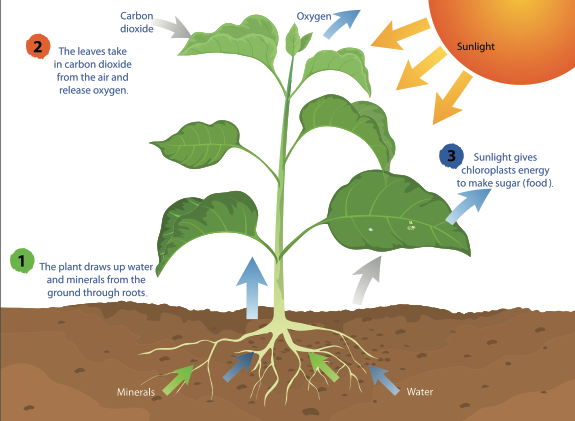
Photosynthesis is a HUGE part of the world as a whole. Without it, plants would die, leading to global pollution, and ultimately the end of the human species. Starting with solar rays, the sun transmits rays down to Earth. Here on Earth plants receive those solar rays and hold on to them until it receives the other two factors of photosynthesis. The plants need water for photosynthesis, they could get it from the ground, rain, or water sources. Finally plants need gases, such as carbon dioxide in photosynthesis. They will absorb some of the CO2 in the air around them. Once they have all three of those factors, they combine them. They use the solar rays to create a chemical reaction to break down the water and gases into molecules, then they take all three of those factors and combine them to make glucose (food).
Similar to the process of photosynthesis, scientists at the University of Zurich have found an "artificial photosynthesis." They take indium based quantum dots and add zinc sulfide to create nanoparticles. They then take those nanoparticles, and mix them with sun rays and water to create a sustainable version of hydrogen. They then take that hydrogen and tamper with it to create things such as energy and/or methane and gasoline (fuel).
By taking the hydrogen created by the nanoparticles and fusing it with solar ray molecules and water molecules, we can make a sustainable source of methane and gasoline fuels, or just energy to be used for powering many different things. Studies have shown that the mixture of indium based quantum dots and a thin layer of zinc sulfide, is not only more sustainable and eco-friendly, but they are also very efficient when it comes to making hydrogen. Much more efficient then other sources of hydrogen and energy created by quantum dots.
This sustainable source of energy has so much potential to the future of the world. This process can be used to make a more sustainable and eco friendly alternative in many areas. Many scientists have said that "water-solable" and "biocompatible" quantum dots is a key to future success in many fields. They could be used for a more effective way of biomass-to-hydrogen sources of energy. They can also be used to make many different materials. The program behind this discovery (LightCHeC) is looking to make many other different molecules and other ways to use solar energy in chemical bonding to make a better future.

https://ssec.si.edu/stemvisions-blog/what-photosynthesis
https://www.sciencedaily.com/releases/2018/10/181001101929.htm
Photosynthesis is a HUGE part of the world as a whole. Without it, plants would die, leading to global pollution, and ultimately the end of the human species. Starting with solar rays, the sun transmits rays down to Earth. Here on Earth plants receive those solar rays and hold on to them until it receives the other two factors of photosynthesis. The plants need water for photosynthesis, they could get it from the ground, rain, or water sources. Finally plants need gases, such as carbon dioxide in photosynthesis. They will absorb some of the CO2 in the air around them. Once they have all three of those factors, they combine them. They use the solar rays to create a chemical reaction to break down the water and gases into molecules, then they take all three of those factors and combine them to make glucose (food).
Similar to the process of photosynthesis, scientists at the University of Zurich have found an "artificial photosynthesis." They take indium based quantum dots and add zinc sulfide to create nanoparticles. They then take those nanoparticles, and mix them with sun rays and water to create a sustainable version of hydrogen. They then take that hydrogen and tamper with it to create things such as energy and/or methane and gasoline (fuel).
By taking the hydrogen created by the nanoparticles and fusing it with solar ray molecules and water molecules, we can make a sustainable source of methane and gasoline fuels, or just energy to be used for powering many different things. Studies have shown that the mixture of indium based quantum dots and a thin layer of zinc sulfide, is not only more sustainable and eco-friendly, but they are also very efficient when it comes to making hydrogen. Much more efficient then other sources of hydrogen and energy created by quantum dots.
This sustainable source of energy has so much potential to the future of the world. This process can be used to make a more sustainable and eco friendly alternative in many areas. Many scientists have said that "water-solable" and "biocompatible" quantum dots is a key to future success in many fields. They could be used for a more effective way of biomass-to-hydrogen sources of energy. They can also be used to make many different materials. The program behind this discovery (LightCHeC) is looking to make many other different molecules and other ways to use solar energy in chemical bonding to make a better future.


https://ssec.si.edu/stemvisions-blog/what-photosynthesis
https://www.sciencedaily.com/releases/2018/10/181001101929.htm

Comments
Post a Comment