Thermodynamics and How It's Formed
By: Aidan Ryder
What are thermodynamics? Thermodynamics is a separate branch of physics that is caused by a mixture of chemicals. Broadly, thermodynamics is the science that deals with the relationships of heat and forms of energy. It mainly is classified as thermal energy, but can be transferred into all different types. While thermodynamics can be established as many different forms, one main focal point are chemical reactions. One certain chemical reaction includes two separate chemicals. When the chemicals fuse, heat arises. These chemicals are hydrochloric acid and sodium hydroxide.
The first chemical included in this chemical reaction is hydrochloric acid. Hydrochloric acid is a mixture that includes hydrogen chloride and water. When hydrogen chloride is added to water, hydrochloric acid is formed. As well as being a chemical in this chemical reaction, there are many other uses for it. The three main objects this chemical is used for are batteries, photo-flash bulbs, and fireworks. As somebody can notice, many "flashy" things use hydrochloric acid. Fireworks and photo-flash bulbs use hydrochloric acid to give off high amounts of light. These formulas for high amounts of light could also be formed into heat.
The second chemical used in this chemical reaction is sodium hydroxide. Sodium hydroxide is simply a mixture of a metal ion, sodium, and a nonmetal ion, hydroxide. This chemical is mainly used for everyday things like drinking water and paper, but also doubles as a use for cleaning supplies. This chemical is used to make some detergents, soaps, and drain cleaners. For all anybody knows, it could be made out of everyday clothes, as it is also used in certain fabrics. Overall, sodium hydroxide also has many unique uses.
What is the future of thermodynamics? Well, I believe thermodynamics to be used for many things. As its main topic is heat, I believe it will be used as a way to provide heat in colder environments. Somebody could be holding a packet with a divider and when they split the divider, the two chemicals rush together and provide heat. If there are ways to split the hydrochloric acid and sodium hydroxide apart, and they could be reused for heat, there could be an endless supply of warmth to the entire world. Jackets and pants could hold a mixture of the chemicals and provide heat in very low conditions. In conclusion, I believe thermodynamics should be looked into further and should be experimented on more often to provide for the world.
Links:
https://www.livescience.com/50776-thermodynamics.html
https://www.tn.gov/health/cedep/environmental/environmental-health-topics/eht/sodium-hydroxide.html
https://courses.lumenlearning.com/introchem/chapter/the-three-laws-of-thermodynamics/
The first chemical included in this chemical reaction is hydrochloric acid. Hydrochloric acid is a mixture that includes hydrogen chloride and water. When hydrogen chloride is added to water, hydrochloric acid is formed. As well as being a chemical in this chemical reaction, there are many other uses for it. The three main objects this chemical is used for are batteries, photo-flash bulbs, and fireworks. As somebody can notice, many "flashy" things use hydrochloric acid. Fireworks and photo-flash bulbs use hydrochloric acid to give off high amounts of light. These formulas for high amounts of light could also be formed into heat.
The second chemical used in this chemical reaction is sodium hydroxide. Sodium hydroxide is simply a mixture of a metal ion, sodium, and a nonmetal ion, hydroxide. This chemical is mainly used for everyday things like drinking water and paper, but also doubles as a use for cleaning supplies. This chemical is used to make some detergents, soaps, and drain cleaners. For all anybody knows, it could be made out of everyday clothes, as it is also used in certain fabrics. Overall, sodium hydroxide also has many unique uses.
What is the future of thermodynamics? Well, I believe thermodynamics to be used for many things. As its main topic is heat, I believe it will be used as a way to provide heat in colder environments. Somebody could be holding a packet with a divider and when they split the divider, the two chemicals rush together and provide heat. If there are ways to split the hydrochloric acid and sodium hydroxide apart, and they could be reused for heat, there could be an endless supply of warmth to the entire world. Jackets and pants could hold a mixture of the chemicals and provide heat in very low conditions. In conclusion, I believe thermodynamics should be looked into further and should be experimented on more often to provide for the world.
Links:
https://www.livescience.com/50776-thermodynamics.html
https://www.tn.gov/health/cedep/environmental/environmental-health-topics/eht/sodium-hydroxide.html
https://courses.lumenlearning.com/introchem/chapter/the-three-laws-of-thermodynamics/

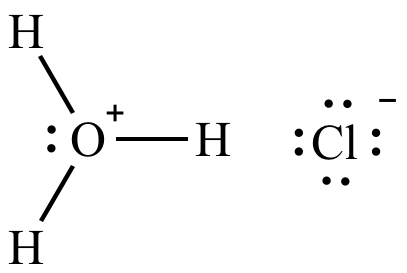


Comments
Post a Comment